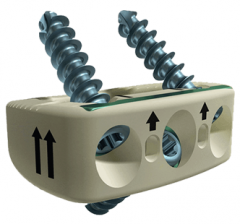
Tritanium C Anterior Cervical Cage
Category:
Interbody Devices
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing






























 Loading...
Loading...