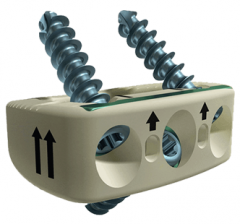
CASCADIA™ Lateral 3D Interbody System
Category:
Minimally Invasive, Interbody Devices
The CASCADIA Lateral 3D Interbody System includes a full range of implant sizes designed to accommodate the vertebral anatomy. This system is designed to work with the RAVINE Lateral Access System, offering a full line of instrumentation for the direct lateral transpsoas approach. Lamellar 3D Titanium Technology incorporates 500 um longitudinal channels throughout the implant, which in conjunction with transverse windows, create an interconnected lattice designed to allow for bony integration. [1]






























 Loading...
Loading...